At Adelphi, we advocate for drug development to be carried out within the final drug packaging from day one.
In this series, we are working on the assumption that your drug will be used in a parenteral application and will ultimately be packed in a vial closed with a stopper and an overseal.
This article focuses on selection of the right aluminium seal or vial cap for your parenteral drug package.
So, let’s get started…
1. Do you have the right seal design?
Firstly, you need to be aware that aluminium seals come in various designs. Each of these variations has a purpose, whether that relates to function, ease-of-use or simply cost considerations.
Most importantly, you will need to ensure the seal you choose is suitable for packaging a parenteral drug. The core purpose of the seal in this instance is to secure the stopper to the vial and prevent removal – maintaining the sterility of the contents. In addition, it should allow access for needle penetration to the stopper, so the drug can be drawn up from the vial immediately prior to administration.

The lowest-cost seal options are made from a lacquered aluminium and feature a central hole for needle insertion.
For additional cleanliness, you might prefer to use a seal or vial cap with a plastic disc covering the aluminium ferrule (or skirt). This disc can be flipped off to reveal the injection site on the stopper, ready for needle insertion.
2. Does it provide good container closure integrity?
If you’ve read our article, 5 Things to Consider when Choosing a Rubber Stopper for Drug Development you’ll already know the importance of good CCI (container closure integrity) in maintaining the sterility of your final drug product.
An injection vial is not considered to be closed until it has been sealed with a secondary closure. This means that the vial cannot leave a controlled environment until the stopper is secured onto the vial. It effectively prevents removal of the stopper. In conjunction with the vial, that should provide the appropriate level of CCI.
Find out more about CCI testing from our partner West Pharmaceutical Services.
3. Do you have the correct crimping equipment?
Traditionally, an injection vial would be sealed with an . This seal has an aluminium skirt, which is crimped under the neck of the injection vial.
Over the years, the pharmaceutical packaging industry has developed and improved aluminium seal design – from the basic ‘seal with a hole’ (still in existence today) to more user-friendly options with plastic discs covering the needle puncture site.
Typically for a parenteral application, we would recommend the use of a ‘Flip Off’ seal. This is an aluminium crimp seal featuring a plastic disc which can be ‘flipped off’ to allow access for needle insertion, whilst maintaining the integrity of the container-closure system.
It is important to note that different seal types require different crimping equipment. For example, a simple aluminium seal will need a different crimping head to a seal with a plastic disc.
Find out more about the different types of crimp seals and how they are used.
Crimping equipment comes in many forms, from manual to fully automated. You can purchase handheld crimping tongs, semi-automatic and automatic crimping equipment from our sister company, Adelphi Manufacturing.
A new alternative to aluminium crimp seals are innovative push-fit vial caps, such as the RayDyLyo vial cap. These can achieve vial closure manually without the need for additional equipment. Push-fit vial caps are pre-fitted with a stopper, providing a convenient one-step solution.
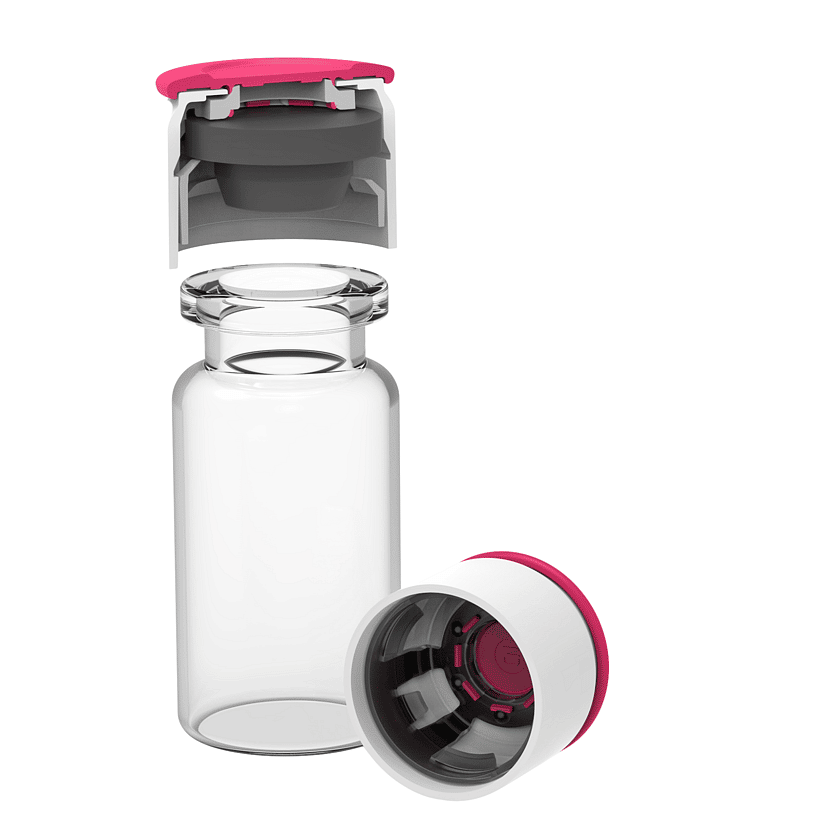
4. Will your drug be marketed in the US?
It is important to ensure your drug packaging meets the criteria of the relevant pharmacopoeia for the region your product will be marketed. The three main pharmacopoeias used globally are EP (European), JP (Japanese) and USP (United States).
You will also want to be aware of other regulatory and advisory bodies, such as the MHRA in the UK, the FDA and the World Health Organization.
In 2010, the FDA released a statement on the use of overseals for parenteral products. It stated that, as of 1st December 2013, no non-essential text should be present on the disc of an overseal. This area should be free of text, leaving the area available for safety-related labelling should it be necessary. That includes the instructional ‘Flip Off’ text that appears on some seal variations.
USP General Chapter <1> Injections states “Only cautionary statements are to be printed on the ferrules and cap overseals of vials containing an injectable drug product. A cautionary statement is one intended to prevent an imminent life threatening situation if the injectable drug is used inappropriately. Examples of such statements are the following: “Warning”, “Dilute Before Using”, “Paralyzing Agent”, “I.M. Use Only”, “Chemotherapy”, etc.
The printing must be in contrasting color and conspicuous under ordinary conditions of use. The cautionary statement may be printed solely on the ferrule, provided the cap overseal is constructed so as to allow the cautionary statement below to be readily legible.”
To support our many customers, either based in the US or manufacturing drugs for use in the US, we now stock only Flip Off crimp seals without text, suitable for use in the US and globally.
Please note: we are still able to supply Flip Off seals with the Flip Off text for those who prefer to use this design for legacy reasons.
5. Does it provide sterility?
Question number one in this article referred to seal design. We highlighted that some seal designs offer additional protection from dust and dirt.
If you are planning to fill aseptically, then you’ll need to go a step further and use an RTU (ready-to-use) closure. In this category, you might look at a sterilised aluminium crimp seal such as West CCS seals They feature a plastic Flip Off disc for protection and tamper evidence, and are supplied clean and certified sterilised.
Alternatively push-fit vial caps, such as the RayDyLyo® vial cap, are also available sterile and ready to use.
RTU options such as these are especially desirable in light of recent updates to GMP Annex 1.
In Conclusion
Selecting the appropriate overseal or vial cap for parenteral drug packaging is crucial for ensuring product safety, integrity and regulatory compliance. Understanding how the vial, stopper and secondary closure work together will help you avoid unnecessary problems down the line.
Considerations include CCI, regulatory compliance, crimping equipment and whether you require sterility. Ready-to-use closures offer peace of mind and compliance with evolving GMP standards. These include sterilised aluminium crimp seals and sterile push-fit vial caps,
By carefully considering each aspect of overseal or vial cap selection, pharmaceutical companies can contribute to the overall success and safety of their parenteral drug products – aligning with Adelphi's commitment to excellence in pharmaceutical packaging.
Whilst we have dedicated this series to parenteral drugs packed in a sealed vial for later administration by needle, the same principle applies for all drug development. You should consider the packaging as a key element of your drug offering. In short, give it proper consideration from day one.



