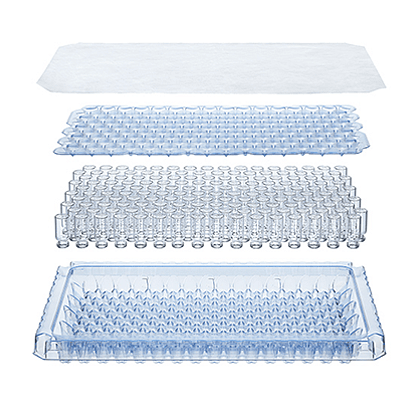
Product Details
Clean, sterile and depyrogenated, SCHOTT Pharma injection vials are processed by Pyrofree® and supplied in vacuum-packed bricks, ready for immediate integration into sterile pharmaceutical filling lines.
Product Applications
These sterile, ready-to-fill vials are ideal for anyone working in sterile drug production, including pharmaceutical and biologics manufacturers, contract manufacturers (CMOs and CDMOs), and research and development organisations. Featuring high quality SCHOTT Pharma Type I glass vials, they offer excellent chemical resistance and are compliant with all the major pharmacopoeia for parenteral applications.