SCHOTT Pharma
SCHOTT Pharma is an industry pioneer specialising in pharmaceutical packaging solutions. With over 100 years of experience, they manufacture premium glass and polymer containers, including syringes, vials and cartridges that are trusted worldwide.

Precision, quality and innovation
SCHOTT Pharma's dedication to precision, quality and innovation ensures the safe delivery of critical healthcare treatments.
We partner with SCHOTT Pharma to help our customers find the best solutions for their packaging needs including sensitive medicines. Their best in class packaging solutions provide the reliability and safety that meets stringent regulatory standards that are essential for high-value pharmaceuticals and biologics.

Why We Partner with SCHOTT Pharma
SCHOTT Pharma's commitment to excellence matches our own. Together, we offer packaging solutions that protect products, enhance shelf life and support regulatory compliance. This partnership allows us to meet the evolving needs of pharmaceutical manufacturers while maintaining the highest levels of quality.

What SCHOTT Pharma brings to you:
- Premium glass and polymer syringes, vials and cartridges
- Uncompromising safety and quality standards
- Expertise in handling sensitive, high-value medicines
- Innovative solutions tailored to formulation challenges
- Global supply capability with consistent quality assurance
View products
View more products-

-
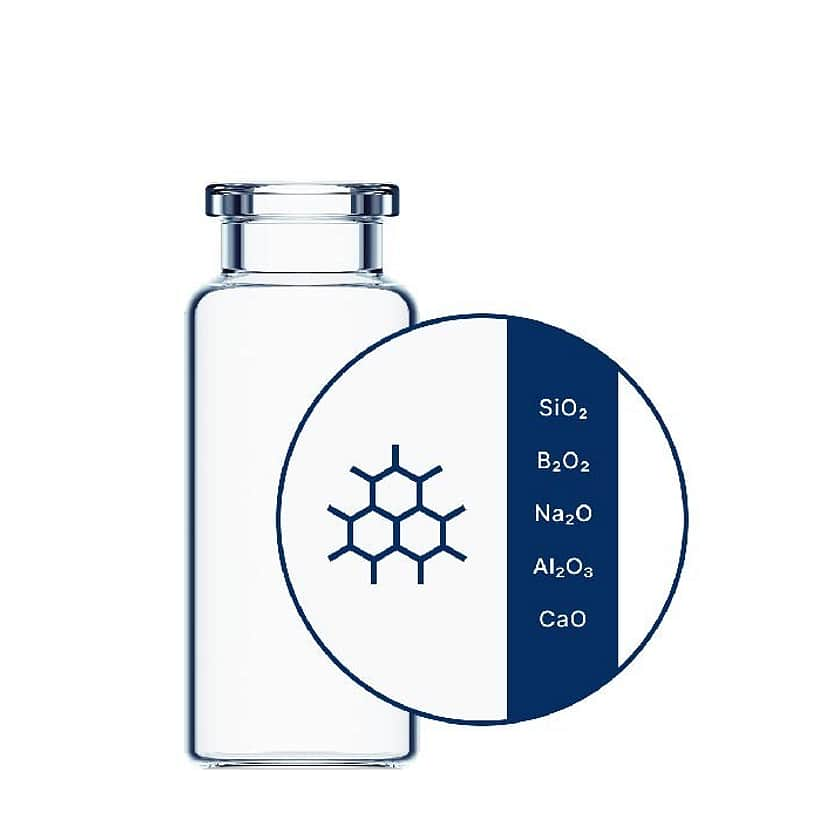
EVERIC® pure vials
EVERIC® pure vials ensure drug stability with their ultra-pure inner surface. See product -

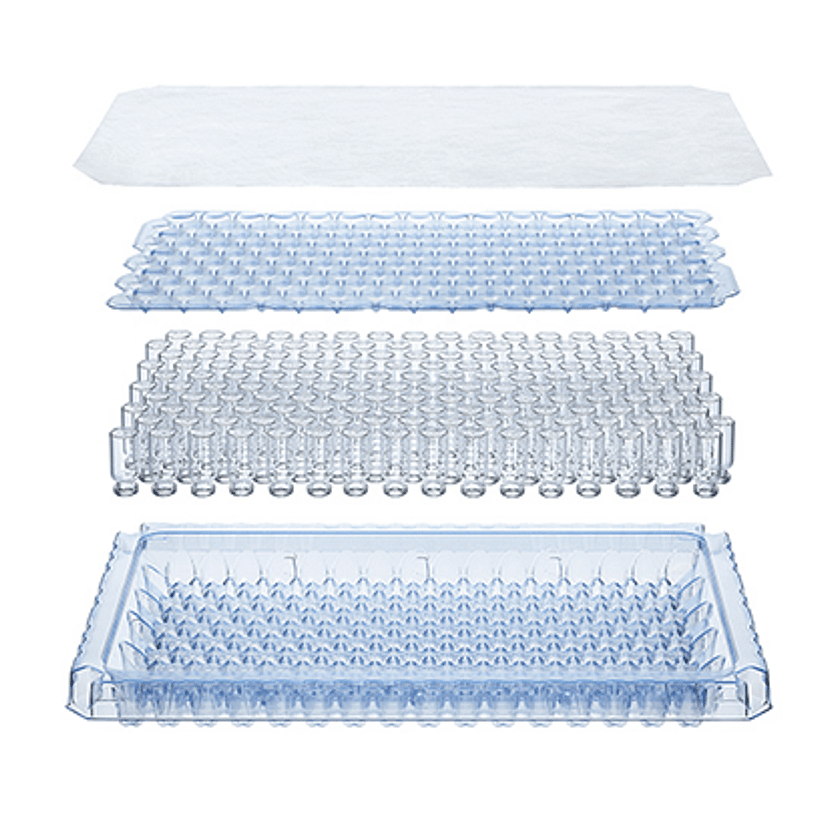
adaptiQ® Sterile Vials in Cup Nest
Sterile and ready-to-use vials in a Cup Nest from SCHOTT Pharma. See product -
-

EVERIC® plus vials
Featuring an SiO2 coating, providing an inert container for sensitive drugs. See product -
-
-

Glass Cartridges
Type I borosilicate glass cartridges for auto-injectors and wearable devices. See product
