What is Annex 1?
Annex 1 is a Good Manufacturing Practice (GMP) regulation. Submitted in 1971, it’s designed to provide both guidance and certification for manufacturers to produce goods in safe and sterile environments. This regulation refers to the manufacture of sterile products, cleanroom classification, monitoring and personnel gowning. Annex 1 is also used to support the manufacture of other goods where the reduction of contamination is considered necessary.
In August 2023, Annex 1 was revised and resubmitted, in line with new ISO regulations and procedures. This sparked wide debate across the industry on how it would influence future procedures – not to mention the financial impact on smaller-scale projects. Despite this, there are areas that leave space for interpretation.
With its first update since 2008, Annex 1 has several new changes that will significantly impact the production of sterile products. Overall, it is there to provide manufacturers with the necessary validation and certification to demonstrate that procedural protocol has been followed – and is in line with industry expectations. In this article, we discuss how Annex 1 will affect pharmaceutical manufacturers in the future. We’ll also explore how your choice of primary packaging could help ease the journey to compliance.
How does Annex 1 affect the pharmaceutical industry?
Although Annex 1’s changes have been widely debated, they come at a pivotal time for the pharmaceuticals and biotech industries. Technological advancement in medicine is ever evolving and new sectors such as biotech and nuclear medicine are continuing to grow.
The latest Annex 1 updates provide much needed guidance and obligatory safety procedures to both serve and protect pharmaceutical companies and patients. This includes:
- Media Fill: Otherwise known as Aseptic Process Simulation (APS). This is where a liquid composed of specific chemicals is used to fill all sides and areas of a sterile container and then sealed within. It is then incubated and inspected over a period to see whether a product is contaminated during an aseptic procedure. Annex 1 has now made this procedure mandatory – not just for liquid-based products, but also for non-filtered formulations, sterile powders and lyophilised (freeze-dried products).
- Cleanrooms: (particulates): Annex 1 will now mandate that cleanroom particles are monitored down to between 5.0 - 0.5 micrometres.
- Barrier technologies: This includes machines such as isolators, filtered vacuums and restricted-access barrier systems. They will now be required to provide suitable protection for housing stable Grade A environments.
- Disinfection: Annex 1 has made it compulsory to frequently clean, both pre- and post-production. The aim being to achieve an above satisfactory level of contamination control. This process will need to be documented, monitored and later validated within regulation to achieve full compliance.
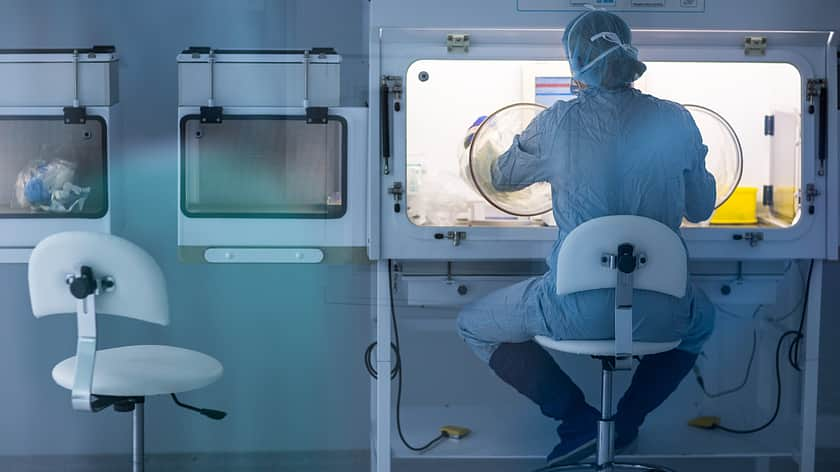
With the above updates, more rigorous procedures must be followed. That might require investment in new equipment, which smaller drug companies may struggle to finance. This could lead to certain process being outsourced to ensure compliance with GMP Annex 1 and ISO11737 regulations.
Mitigating the impact of Annex 1
The new regulations have presented a challenge for smaller-scale projects. Ever more thorough procedures, additional personnel and investment in adequate machinery could be seen as insurmountable obstacles. However, there are solutions to ease the way to compliance.
Ready-to-use primary packaging components provide peace of mind in adhering to the Annex I guidance. Vials and stoppers can be supplied sterile and ready-to-use in an aseptic environment. This offers a cost-effective alternative to sterilising your packaging in-house.
RTU vials and stoppers are supplied with full sterility certification, conforming to industry-wide regulations. They are a great solution for projects where resources don’t allow for inhouse sterilisation.
An example of Sterile RTU vials is the SCHOTT adaptiQ® range which is supplied in a choice of secondary packaging formats – clean, sterile and pyrogen-free. AdaptiQ® sterile vials can be processed manually or on a range of new and existing fill-and-finish lines. Rubber stoppers can also be supplied sterile and ready-to-use for incorporation into your aseptic manufacturing process, with examples including West’s NovaPure Rubber Stopper.

For more information on SCHOTT adaptiadaptiQ® Sterile Vials in Cup NestQ® vials or West’s NovaPure Rubber Stoppers, please get in touch with our team, and we can provide globally trusted technical advice and support.
Why the Annex 1 revisions are a good measure despite their impact on the industry
In conclusion, Annex 1's recent revisions reflect the evolving landscape of the pharmaceutical industry, emphasizing safety and sterility in an era of increased manufacturer accountability. While these changes may present challenges, alternatives like sterile RTU vials offer practical solutions for pharmaceutical research projects, reducing financial burdens and ensuring compliance with the updated regulations.
If you want to know more about sterile packaging, browse our sterile packaging range and discover our full offering of RTU packaging.
